Catalysts for Asymmetric Reductive Amination
–Ir-PSA series–
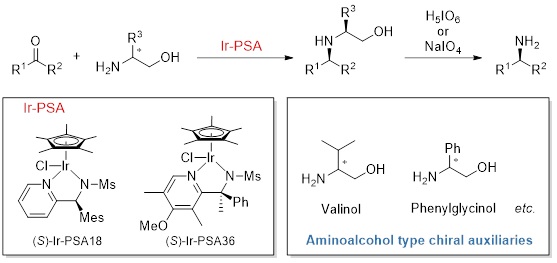
Ir-PSA
Kanto Chemical has developed novel asymmetric reductive amination catalysts, Ir-PSA for preparation of optically active amines.
Optically active amines are important compounds such as intermediates of pharmaceuticals. Conventional methods for synthesis of optically active amines include optical resolution of racemic amines and enantioselective or diastereoselective reduction of pre-prepared imines. These were not efficient methods due to low yield, long steps and poor functional group tolerance. Therefore, we improved our original reductive amination catalysts (Ir-PA, Ir-QN) into asymmetric catalysts (Ir-PSA) and developed an efficient method for preparation of optically active amines. In this method, combination of chiral Ir-PSA and an inexpensive aminoalcohol-based chiral auxiliary enables efficient asymmetric reductive amination of ketones to afford corresponding optically active amines in high yield and high stereoselectivity. It is especially effective for amines that are difficult to obtain by conventional asymmetric synthesis. And the aminoalcohol moiety can be easily and quantitatively removed under mild oxidative conditions. This method is a practical reaction with excellent functional group tolerance. Ir-PSA are available in both S and R enantiomers. Ir-PSA18 is especially effective for the asymmetric reductive amination of phenylacetones, and Ir-PSA36 is effective for the 2-tetralones.
Reaction examples by Ir-PSA18
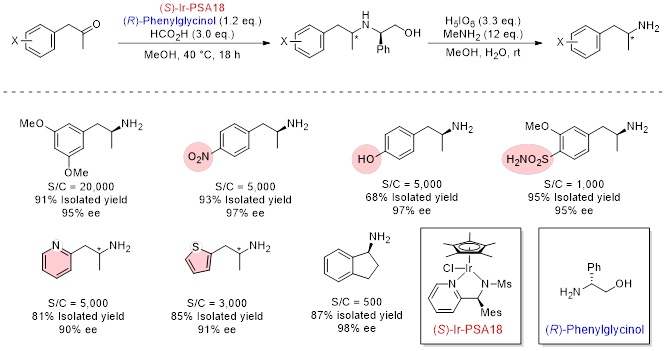
Reaction examples by Ir-PSA36
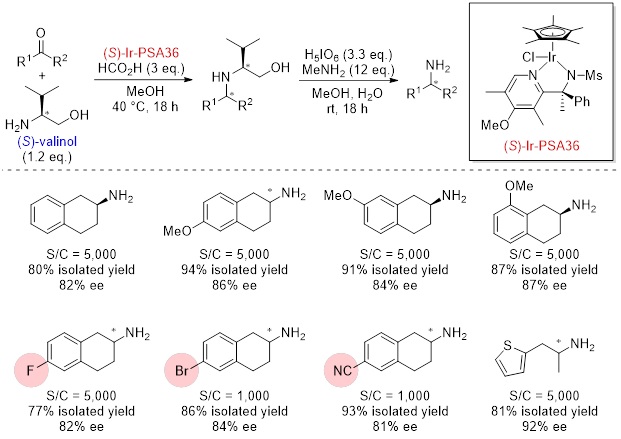
Comparison with other asymmetric reductive aminations
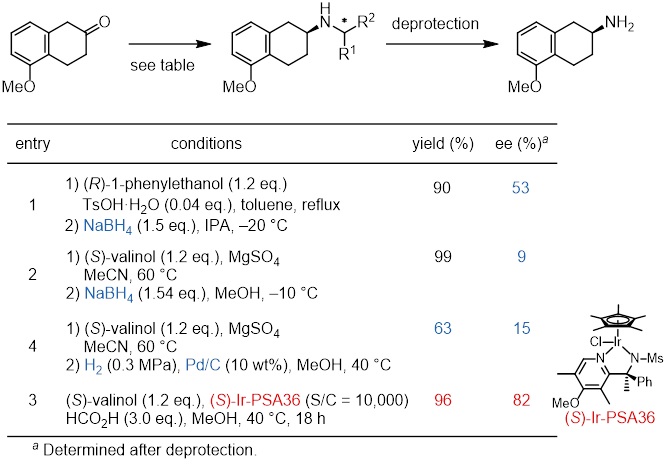
This method exhibits superior stereoselectivity compared to sodium borohydride reduction and Pd catalytic hydrogenation.
Application to Rotigotine intermediate synthesis
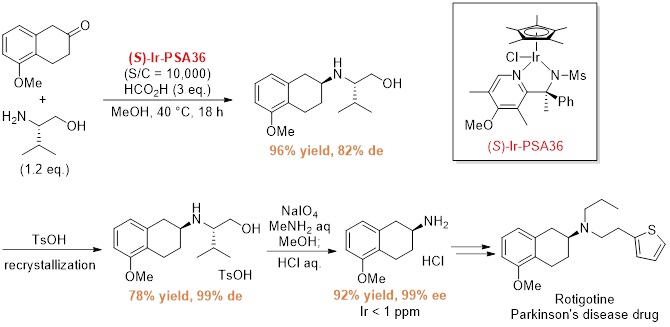
The optical purity can be easily increased by recrystallization of the diastereomeric intermediate.
Application to Tamsulosin intermediate synthesis
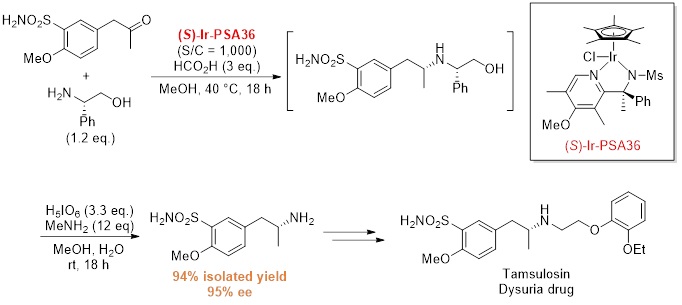
Process shortening and cost reduction can be expected in optically active amine synthesis.
Product list
| Product | Product | Grade | Product No. | Package |
|---|---|---|---|---|
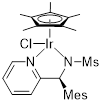 |
( S)-Ir-PSA18 Chloro[( S)-N-{1-(4-pyridin-2-yl(2,4,6-trimethylphenyl)methyl}methanesulfonamidato](pentamethylcyclopentadienyl)iridium(III) |
for asymmetric synthesis | 07060-68 | 100mg |
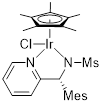 |
(R)-Ir-PSA18 Chloro[(R)-N-{1-(4-pyridin-2-yl(2,4,6-trimethylphenyl)methyl}methanesulfonamidato](pentamethylcyclopentadienyl)iridium(III) |
for asymmetric synthesis | 07071-68 | 100mg |
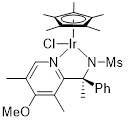 |
(S)-Ir-PSA36 Chloro[(S)-N-(1-(4-methoxy-3,5-dimethylpyridin-2-yl)-1-phenylethyl)methanesulfonamidato](pentamethylcyclopentadienyl)iridium(III) |
for asymmetric synthesis | 07658-68 | 100mg |
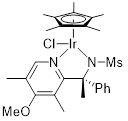 |
(R)-Ir-PSA36 Chloro[(R)-N-(1-(4-methoxy-3,5-dimethylpyridin-2-yl)-1-phenylethyl)methanesulfonamidato](pentamethylcyclopentadienyl)iridium(III) |
for asymmetric synthesis | 07035-68 | 100mg |
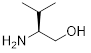 |
L-Valinol | 44078-32 |
25g | |
| 44078-52 | 5g | |||
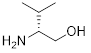 |
(R)-(-)-2-Amino-3-methyl-1-butanol | 42247-2A | 5g | |
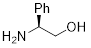 |
(S)-(+)-Phenylglycinol | 30757-1A | 1g | |
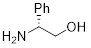 |
D(-)-α- Phenylglycinol | 18382-1A | 5g | |
| NaIO4 | Sodium periodate | Guaranteed reagent for JIS | 37233-00 | 500g |
| 37233-20 | 100g | |||
| 37233-30 | 25g | |||
| H5IO6 | Orthoperiodic acid | Guaranteed reagent | 32061-30 | 25g |
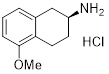 |
(S)-2-Amino-5-methoxytetralin hydrochloride | for asymmetric synthesis | 01770-55 | 5g |
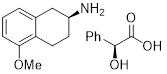 |
(S)-2-Amino-5-methoxytetralin (S)-mandelate | for asymmetric synthesis | 01769-55 | 5g |
Patent application by Kanto Chemical Co.
Application number
- WO2014175267
- JP2022-075375
- JP2022-075379
Related Information
- Brochure:
Iridium Catalyst for Chiral Amine Synthesis - Related Page:
Publications
- Asymmetric Transfer Hydrogenative Amination of Benzylic Ketones Catalyzed by Cp*Ir(III) Complexes Bearing a Chiral N-(2-Picolyl)sulfonamidato Ligand
T.Kawada, K.Yabushita, T.Yasuda, T.Ohta, T.Yajima, K.Tanaka, N.Utsumi, M.Watanabe, K.Murata, Y.Kayaki, S.Kuwata, and T.Katayama
The Journal of Organic Chemistry, 87(13), 8458-8468(2022) - Development of an Efficient Method for the Synthesis of Chiral Amines by Using New Chiral Iridium Catalyst.
T.Kawada, T.Katayama
THE CHEMICAL TIMES, 264, 26-31 (2022).
Contact Us
E-mail Inquiry Form
Telephone