Management systems・Quality assurance(Accreditation・Certification)
The details of each certified management system are listed.
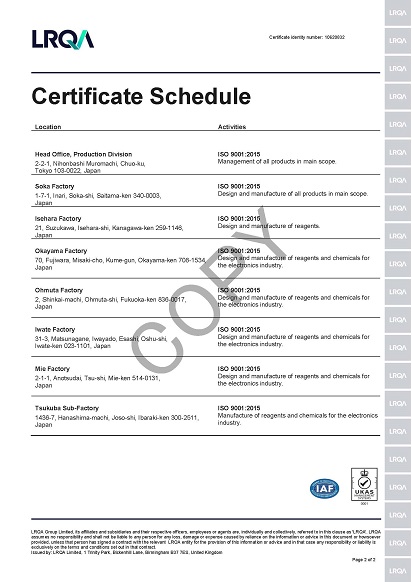
Certified according to ISO 9001(Quality management systems)
For more credible quality management systems and to fully meet customers' requirements, our Soka factory acquired the certification on ISO 9001 in October 1994, making it the first company in Japanese reagent industry. Following this, all other factories were also certified, too. We will continue further improvement our own quality management systems and supply more reliable products.
Outline of Certification
| Factory names | The scope of certification | Approval certificate No. |
|---|---|---|
| Soka factory | Design and manufacture of reagents, chemicals for the electronic industry and fine chemicals. | ISO 9001-0077395 |
| Isehara factory | Design and manufacture of reagents, chemicals for the electronic industry | |
| Okayama factory | Design and manufacture of reagents, chemicals for the electronic industry. | |
| Omuta factory | Design and manufacture of reagents, chemicals for the electronic industry | |
| Iwate factory | Design and manufacture of reagents, chemicals for the electronic industry | |
| Mie factory | Design and manufacture of reagents, chemicals for the electronic industry. |
Procedures of certification were changed to the multi site on the head office (The production division) and all the factories in August 2003.
Certified according to ISO 14001 (Environmental management systems)
In July 1998, as a forerunner of reagent industry, the factories of Kanto were certified according to "ISO 14001"for environmental management systems. Giving top priority over environmental protection, all our factories have been improved to ensure the environmental-friendly production processes such as saving energy, economize natural resources and reducing industrial waste. We continue to develop the ways and means for the protecting global environment.
As a reagent supplier, KANTO is also willingly taking effort to supply useful analytical reagents related with our customers' environmental inspection activities,
Outline of Certification
| Factory names | The scope of certification | Approval certificate No. |
|---|---|---|
| Soka factory | Design and manufacture of reagents, chemicals for the electronic industry and fine chemicals. | ISO 14001-0077344 |
| Isehara factory | Design and manufacture of reagents, Microbiological reagents, Biochemical reagents and in vitro diagnostic reagents. | ISO 14001-0077346 |
| Okayama factory | Design and manufacture of reagents, chemicals for the electronic industry. | ISO 14001-0077348 |
| Omuta factory | Design and manufacture of reagents, chemicals for the electronic industry. | ISO 14001-0077347 |
| Iwate factory | Design and manufacture of reagents, chemicals for the electronic industry and fine chemicals. | ISO 14001-0077345 |
| Mie factory | Design and manufacture of reagents, chemicals for the electronic industry. | ISO 14001-0076748 |
[ Certified by : LRQA Limited ]
Certified according to ISO 13485 (Quality management systems on medical devices)
In January 2005, the quality management systems for our products related to in-vitro diagnostics was certified according to ISO 13485, which is an international standard on quality management systems for medical devices. This standard was established based on ISO 9001 by adding up various specific requirements from the aspect of the influence and safety on clinical use.
In order to guarantee the demanding high quality for in-vitro diagnostics, we supply our own products under this internationally standardized quality management systems including the requirements of GMP (Good Manufacture Practice) requested by Ministry of Health, Labor and Welfare.
Outline of Certification
| The name of the company | Kanto Chemical Co., Inc. Life Science Department Isehara Factory |
|---|---|
| Scope of certification | Design and Development, Production and Distribution of Clinical Chemistry Reagents, Immunological Reagents and related products. Import, Production and Distribution of Microbiological Reagents. |
| Date of issue of Certification | January 5, 2005 : Primary audit |
| Certification body | TÜV SÜD Japan Ltd. |
Accredited according to ISO/IEC 17025 (General requirements for the competence of testing and calibration laboratories)
The ISO/IEC 17025 standard establishes requirements for a laboratory management system that combines quality management system requirements with technical requirements. The standard can be thought of as a technically rigorous application of ISO 9001 to a calibration or testing laboratory.
We are proud to be the first reagent company to acquire this certification in Japan.
The certification was issued by The Japan Accreditation Board for Conformity Assessment (JAB) that complies with International Mutual Recognition Arrangement (MRA). We provide customers test report with a ILAC-MRA/JAB logo specifying the "UNCERTAINITY" that proves the accuracy of our test results. The above logo is a symbol that ensures importing countries to grant an equivalent official valuation there to the test report in accordance with MRA.
As one of world-leading chemical analysis institute, we continue to provide reliable test results.
Outline of Accreditation
| The name of laboratory | Kanto Chemical Co., Inc. Soka Factory |
|---|---|
| The date of Accreditation | October 20, 2000 |
| Accreditation body | Japan Accreditation Board (JAB) |
| Certificate No. | RTL00780 |
| The scope of Accreditation |
JIS K 8001 General rule for test methods of reagents annex JA.6 Volumetric solutions |
| JA.6.4 c) [except 5)] EDTA2Na solution Measurement Range 0.01 mol/L or more, 0.1 mol/L or less Sampling:According to Standard Operation Procedure(SO-EE-041) |
|
| JA.6.4 e) [except 4)] : Partial change (minimum limit) Hydrochloric acid Measurement Range 0.05 mol/L or more, 2 mol/L or less Sampling:According to Standard Operation Procedure(SO-EE-041) |
|
| JA.6.4 r):Pertial change (maximum and minimum limit) Sodium hydroxide solution Measurement Range 0.05 mol/L or more, 2 mol/L or less Sampling:According to Standard Operation Procedure(SO-EE-041) |
|
| JA.6.4 ab) [except4),5)] Potassium hydroxide solution Measurement Range 0.1 mol/L or more, 1 mol/L or less Sampling:According to Standard Operation Procedure(SO-EE-041) |
|
| JA.6.4 y) Sulfuric acid Measurement Range 0.05 mol/L or more, 0.5 mol/L or less Sampling:According to Standard Operation Procedure(SO-EE-041) |
|
| JA.6.4 f) 0.1 mol/L Perchloric acid in Glacial acetic acid Sampling:According to Standard Operation Procedure(SO-EE-041) |
|
|
JA.6.4 g) Partial change (maximum limit) |
|
|
JA.6.4 l) 1) 0.05 mol/L Sodium oxalate solution |
|
|
JA.6.4 n) 0.1 mol/L Silver nitrate solution |
|
|
JA.6.4 n) : Partial change (the test method of the Silver nitrate solution) |
|
|
JA.6.4 t) 2) 0.1mol/L Sodium thiosulfate solution |
|
|
JA.6.4 w):Partial change (Potentiometric titration) |
|
|
Applied item goods |
1) 0.1 mol/L Disodium dihydrogen ethylenediamine tetraacetate solution (capacity 500 mL) 2) 0.05 mol/L Disodium dihydrogen ethylenediamine tetraacetate solution (capacity 500 mL) 3) 0.01 mol/L Disodium dihydrogen ethylenediamine tetraacetate solution (capacity 500 mL) 4) 1 mol/L Hydrochloric acid (capacity 500 mL) 5) 0.5 mol/L Hydrochloric acid (capacity 500 mL) 6) 0.1 mol/L Hydrochloric acid (capacity 500 mL) 7) 0.05 mol/L Hydrochloric acid (capacity 500 mL) 8) 1 mol/L Sodium hydroxide solution (capacity 500 mL) 9) 0.1 mol/L Sodium hydroxide solution (capacity 500 mL) 10) 0.5 mol/L Sulfuric acid (capacity 500 mL) 11) 0.25 mol/L Sulfuric acid (capacity 500 mL) 12) 0.05 mol/L Sulfuric acid (capacity 500 mL) 13) 0.1 mol/L Perchloric acid-acetic acid solution (capacity 500 mL) 14) 0.1 mol/L Potassium permanganate solution (capacity 500 mL) 15) 0.02 mol/L Potassium permanganate solution (capacity 500 mL) 16) 0.05 mol/L Sodium oxalate solution (capacity 500 mL) 17) 0.1 mol/L Silver nitrate solution (capacity 500 mL) 18) 0.1 mol/L Sodium chloride solution (capacity 500 mL) 19) 0.1 mol/L Sodium thiosulfate solution (capacity 500 mL) 20) 0.05 mol/L Iodine solution (capacity 500 mL) |
Accredited according to JCSS (The traceability system to National Measurement Standards)
Measurement Law Traceability System was established for the purpose of ensuing high precision measurement and the confidence of quality control on industrial production process. The accreditation of JCSS (Japan Calibration Service System) verifies and guarantees that the measurement standards calibrated by the qualified company are precision and traceable to National Standards.
http://www.nite.go.jp/iajapan/jcss/outline/index.html
To clearly identify a company's product quality, technology and business spirits, current industry requires many types of certification.
For instance, ISO 9001 for quality managements, ISO 14001 for environmental managements, ISO/IEC 17025 for precision analysis and measurement, GLP (Good Laboratory Practice) and GMP (Good Manufacturing Practice) for quality and hazard risk management are the basic selection as the globally harmonized system for this purpose.
All of those standards also require the consistent traceability system on their testing and measurement practices.
As a JCSS-accredited corporation, we supply various kinds of standard solutions traceable to the national measurement standards of pH, metal, non-metal and volatite organics and issue certificates with "JCSS" logo symbolizing precision. This greatly contributes to accuracy and reliability of tests and calibrations in the world industries.
We are also accredited by IAJapan, that is an affiliate of MRA between ILAC( International Laboratory Accreditation Cooperation) and APAC(Asia Pacific Accreditation Cooperation) the logo, therefore, is internationally recognized.
- IAJapan: An independent organization to manage accredited programs of corporations practicing tests and calibrations in National Institute of Technology and Evaluation (NITE).
-This accreditation demonstrates technical competencs for a defined scope and the operation of a laboratory quality management system. ISO 17034:2016 includes the requirements of ISO/IEC 17025:2017 for testing, calibration and related activities in the assignment of property values to certified reference materials.
Outline of Accreditation
| Product | Number of products |
Accredited corporation |
|---|---|---|
| pH standard solution (first class) |
2 | Isehara Factory (Accredited number:0015) |
| pH standard solution (second class) |
12 | |
| Metal standard solution | 66 | Soka factory (Accredited number:0014) |
| Metal mixture standard solution | 1 | |
| Non-metallic ion standard solution | 16 | |
| Organic compound standard solution | 3 | |
| Organic compound mixture standard solution | 6 |
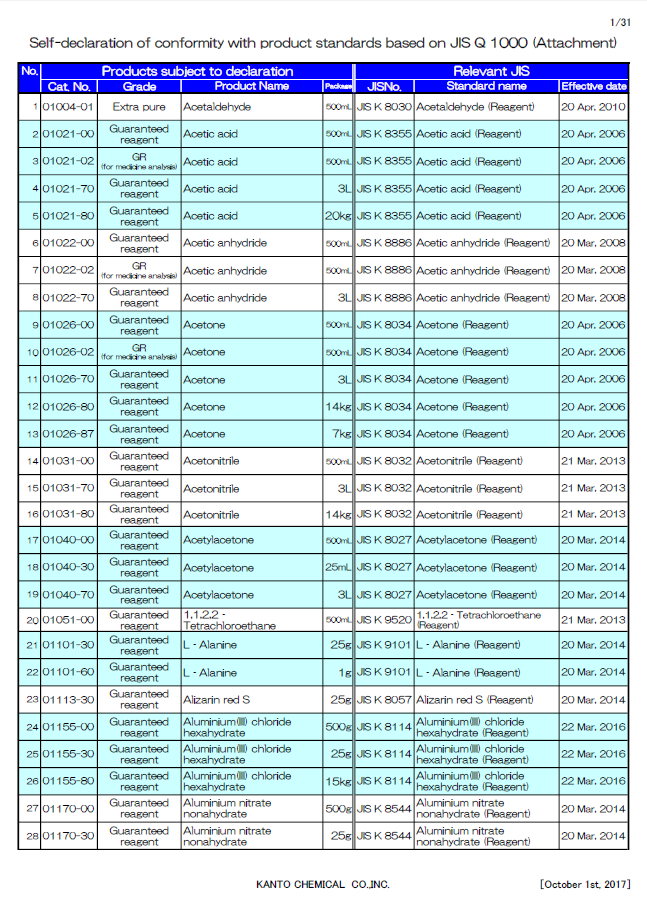
Self-declaration of conformity with product standards based on JIS Q 1000
The basic system for JIS marking was amended in 2004 which included the change from governmental accreditation to private third party certification. Additionally the conventional marking system was removed. Therefore the system provides business entities with free choice of “JIS indication” or “self-declaration of conformity”.
We had adopted the mark of the Japanese Industrial Standard (which is called “JIS mark”) indicated on our products for a long time, however, after the above JIS amendment we decided to adopt “JIS Q 1000 Conformity assessment - Guidelines for suppliers' declarations of conformity with product standards”.
“Suppliers' declarations of conformity” means that a business entity clearly declares and guarantees their products conforming to JIS.
The standard “JIS Q 1000” was enacted in order to ensure the reliability of above. “JIS Q 1000” recommends the ISO9001 and ISO/IEC 17025 for operation.
In addition to the quality assurance system conforming to ISO 9001, we are certified according to ISO/IEC 17025 to demonstrate technical competence of Testing and Calibration of Laboratories with annual assessment by the third party organization. Also, in accord with supply performance of JIS reagents for over 60 years, we follow the raw material, production equipment, production process and the control standards etc. to the new system and we supply the reagents of reliable quality in the same manner.
We strive to work on reinforcement of quality assurance system as heretofore and make further efforts to improve the level of service.
The indication of the catalogue and the label

For the products manufactured based on suppliers' declarations of conformity, “JIS K XXXX” is indicated on the product label and in the catalogue.
■JIS Q 1000 Conformity assessment - Guidelines for suppliers' declarations of conformity with product standards-
The standard specifies the general requirements for suppliers on “Guidelines for suppliers' declarations of conformity with product standards”.