Application examples
The following are some examples of applications in the field of carbon neutrality: Battery research (Lithium ion batteries, Ionic liquid gel electrolytes), carbon dioxide separation and recovery, bioscience and life science (biological sample solvents).
Carbon Neutrality
Recently, the world has become increasingly aware of global warming, environmental issues and sustainability. In accordance with the Paris Agreement adopted in 2015, the world are implementing measures to reduce greenhouse gas emissions. In 2019, the Japanese Cabinet approved a long-term strategy for growth based on the Paris Agreement, which calls for a "Green Growth Strategy Through Achieving Carbon Neutrality in 20501)". In this context, ionic liquids are attracting attention as an environmentally-friendly material with unique characteristics. In this section, we introduce the use of ionic liquids in the field of energy and battery research, and the separation and absorption of carbon dioxide.
Energy / Battery Research
Lithium ion battery
Since the commercialisation of lithium-ion batteries in 1991, they have been continuously improved for use in a wide range of media, including in EV(electric vehicles). A typical lithium-ion battery uses an electrolyte between the positive and negative electrodes, which is charged and discharged by the movement of lithium ions through the electrolyte. However, the electrolyte is often made from flammable organic solvents, which poses a safety issue. Ionic liquids have been considered for use as electrolytes because they are non-volatile, non-flammable and exhibit high ionic conductivity.2)3)
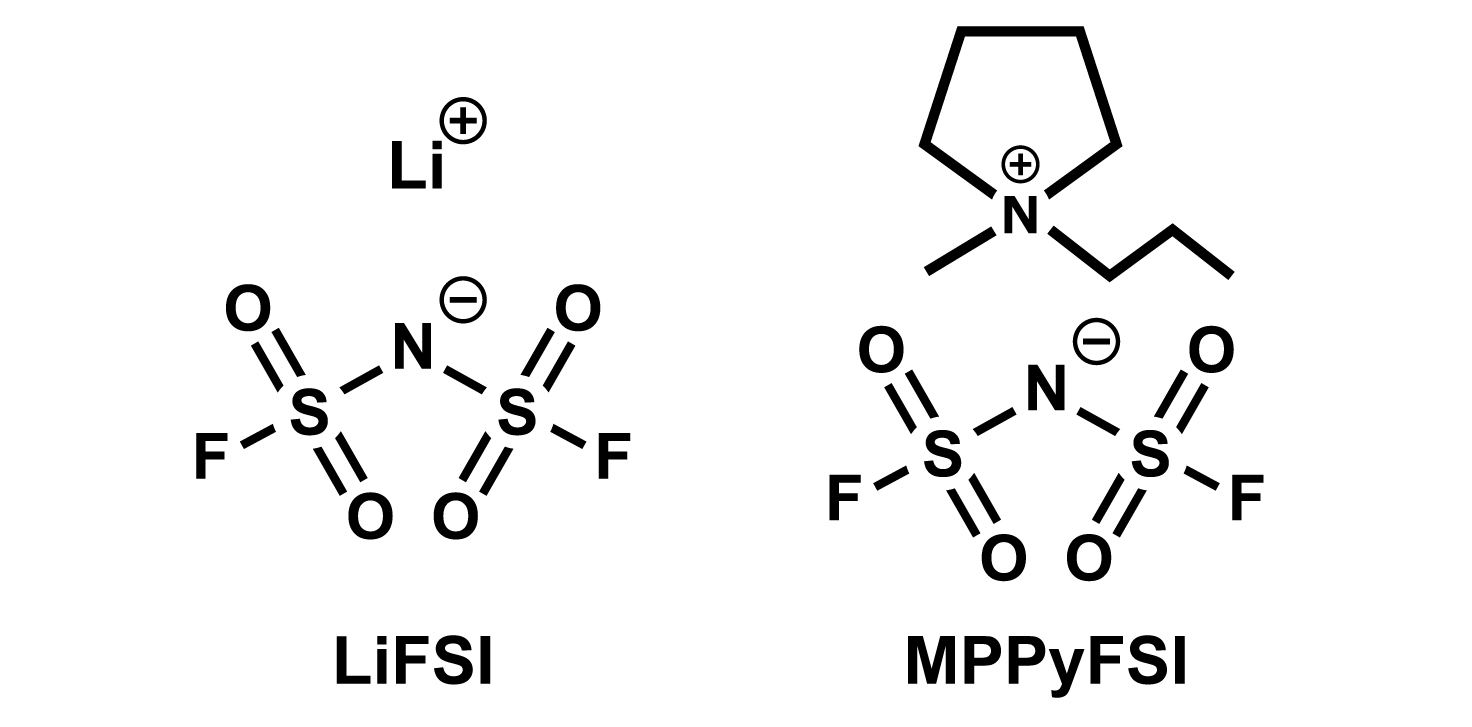
In addition, ionic liquids have almost no vapour pressure and are stable even under high vacuum. Lithium-ion batteries have been developed for “space environment” applications. The battery is made of LiFSI (lithium salt) and MPPyFSI (ionic liquid) and was installed in the small satellite "Hodoyoshi 3 ", which is currently in Earth orbit and has successfully operated the ionic liquid battery as of 2021. The battery is simply reinforced with a laminated outer shell and is able to withstand the harsh environment of space. It was reported that ionic liquid batteries can be small and lightweight and have high battery performance.4)
Ionic liquid gel electrolytes
Ionic liquids show excellent performance as electrolytes thanks to their characteristics, but there are still concerns about leakage. On the other hand, "Ionic liquid gel electrolytes", which are made by gelation of ionic liquids, are expected to be applied to electrochemical devices as gel electrolytes with high safety, and research is being conducted. 5) Various gelation techniques have been investigated, such as cross-linked polymer formation by polymerization, mixing with polymers, low molecular weight gelators, and mixing with solid particles. However, there are some problems such as the significant decrease in ionic conductivity after gelation.6)7) These problems have been improved by the development of new gelation techniques, and it has been reported that ionic conductivity comparable to that of ionic liquids can be maintained.8)9) It has also been reported that gelation and ionic conductivity can be maintained even at concentrations as low as 0.1 to 2.0 wt.% using gelators reported in recent years.10)
Separation and recovery of carbon dioxide
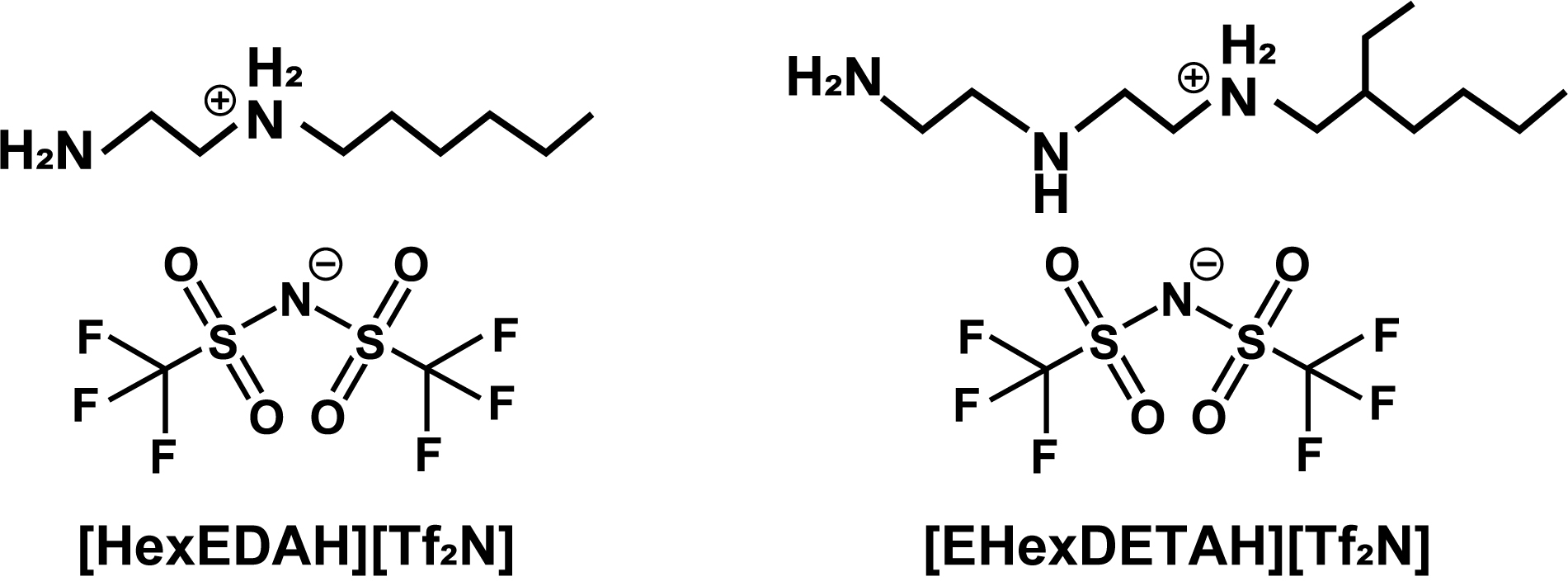
Ionic liquids are known for their ability to absorb acidic gases such as carbon dioxide (CO2) with little or no absorption of gases such as nitrogen or hydrogen, making them suitable for use in carbon dioxide separation and recovery processes. Ionic liquids with fluorine or trifluoromethyl (CF3) anions have a high solubility in CO2. It has been reported that the gas solubility of ionic liquids is more dependent on the anion than on the cation and that they exhibit high solubility thanks to their interaction with CO2.11)12)13) Ionic liquids with an amino group (NH2 group) have also been reported to absorb CO2 as well. Bio-derived amino acid-based ionic liquids with NH2 groups in their anionic moieties are highly reactive, show excellent CO2 absorption and have been reported to absorb CO2 over a wide temperature range.14)15) Although they have been found to absorb a large amount of CO2 at high temperatures, the temperature dependence is small and further high temperature conditions are required in the separation and recovery process. However, the temperature dependence of the absorption is small and the process requires higher temperature conditions. It has been reported that ionic liquids with multiple NH2 groups in the cationic moiety have a large temperature dependence and do not readily absorb CO2 at high temperatures, thus allowing the CO2 to be dissipated by heating and the absorbent to be regenerated.16) Gas absorption using amine solutions is currently used as a CO2 separation technology, but in order to recover the CO2, the gas absorption must be followed by heating and decompression. During the recovery process, the amine solution volatilises, causing losses, whereas ionic liquids, which are difficult to volatilise, can compensate for this drawback. A further advantage is that the performance of ionic liquids is reported to degrade little even after multiple CO2 separation and recovery cycles.15) In addition, membranes containing ionic liquids are being developed for CO2 separation and recovery, which are expected to improve the efficiency of the process as they do not require temperature changes during the separation operation and can continuously separate high concentrations of CO2. Ionic liquid is not volatile, so it can be retained in the membrane and is highly stable at high temperatures and pressures. It is also possible to add CO2/N2 selectivity to the membrane, which is expected to become practical once the thin-film technology is established. 17)18)
Biosciences / Life sciences
While ionic liquids play an essential role in synthetic solvents, extraction solvents and electrochemistry, their unique properties make them attractive for applications in the biosciences and life sciences.
Biorefinery / Biomass dissolution
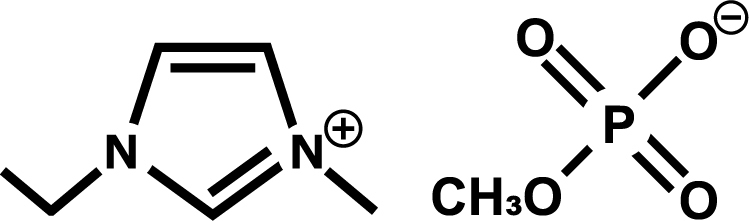
Biomass is a natural polymer found in abundance on earth and is attracting attention as a renewable resource. It has a wide range of applications, including film, fibre and as a raw material for bioethanol. In order to put biomass to practical use, it needs to be dissolved and processed. Since the first report in 2002 on the dissolution of cellulose by ionic liquids, the application of ionic liquids to biomass-related fields has been actively studied. 19) The structure of ionic liquids and their ability to dissolve cellulose have been extensively investigated and it has been found that highly polar ionic liquids consisting of imidazolium cations and phosphite anions can dissolve cellulose under unheated conditions.20) It was found that cellulose could be dissolved under unheated conditions, but that the dissolution capacity was lost when a small amount of water was present. It was therefore necessary to dry the cellulose before dissolving it, and the dissolution process had to be carried out in a dry, enclosed space.
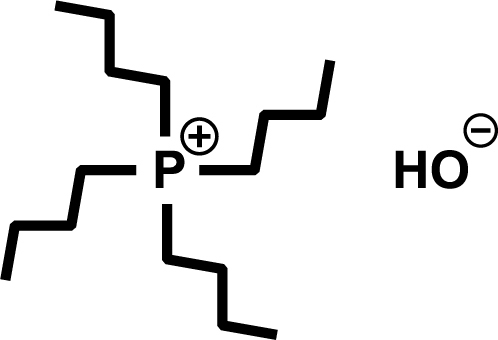
Hydroxides with alkylphosphonium cations have been found to dissolve cellulose even under water-containing conditions, and cellulose dissolution under conditions containing large amounts of water has been reported.21)
Ionic liquids as novel solvents in life science
In the life sciences, organic solvents are often used to cryopreserve biological samples or to dissolve drugs that are insoluble in water. One of the most common organic solvents used in the life sciences is DMSO (dimethyl sulphoxide). DMSO has also been used for many years as it acts as a cryoprotective agent to preserve cells. However, although the toxicity of DMSO is considered to be relatively low among organic solvents, it cannot be completely ignored: DMSO is cell permeable and is known to have a variety of effects on cells. Zwitterionic liquids, ionic liquids containing both cations and anions in the same molecule, hold promise as a new non-aqueous medium for the preservation of biological samples. Carboxylic acid-based zwitterionic liquids, which have a structure similar to that of histidine, exist as liquids at room temperature and have the potential to dissolve cellulose, which has very low solubility in water and DMSO, as a non-aqueous solvent. The same as DMSO, they have also been found to act as solvents for cryoprotective agent and hydrophobic drugs.22)
Reference
- 1) Ministry of Economy, Trade and Industry
- 2) Sakaebe, H., Matsumoto, H., Electrochemistry Communications, 2003, 5, 594-598.
- 3) Watanabe, M., Thomas, L. M., Zhang, S.,Ueno, K., Yasuda, T., Dokko, K., Chemical Review, 2017, 117, 10, 7190–7239.
- 4) Yamagata, M., Tanaka, K., Tsuruda, Y., Sone, Y., Fukuda, S., Nakasuka, S., Kono, M., Ishikawa, M., Electrochemistry, 2015, 83, 918.
- 5) Karuppasamy K., Theerthagiri J., Vikraman D., Yim C.J., Hussain S., Sharma R., Maiyalagan T., Qin J., Kim H.S., Polymers.2020, 12, 918.
- 6) Noda, A.; Watanabe, M. Electrochim. Acta., 2000, 45, 1265.
- 7) Fuller, J.; Breda, A. C.; Carlin, R. T. J. Electrochem. Soc., 1997, 144, L67
- 8) Fujii, K.; Asai, H.; Ueki, T.; Sakai, T.; Imaizumi, S.; Chung, U.; Watanabe, M.; Shibayama, M. Soft Matter, 2012, 8, 1756
- 9) Nagasawa, J., Matsumoto. H., Yoshida. M., ACS Macro Lett., 2012, 1, 1108
- 10) Minakuchi, N., Hoe, K., Yamaki, D., Ten-no, S., Nakashima, K., Goto, M., Mizuhata, M., Maruyama, T., Langmuir, 2012, 28, 9259.
- 11) Aki, S. N.V.K., Mellein, B. R., Saurer, E. M., Brennecke, J. F., J. Phys. Chem.B, 2004, 108, 20355
- 12) Kazarian, S. G., Briscoe, B.J., Welton, T., Chem. Commun., 2000, 2047.
- 13) Seki, T., Grunwaldt, J.-D., Baiker, A., J. Phys. Chem. B., 2009, 113, 114.
- 14) Goodrich, B. F., de la Fuente, J. C., Gurkan, B. E., Lopez, Z. K., Price, E. A., Huang, Y., Brennecke, J. F., J. Phys. Chem. B., 2011, 115, 9140.
- 15) Makino, T., Kanakubo, M., Kamio, E., Takaba, H., Matsuyama, H., Fluid Phase Equilib., 2016, 420, 89.
- 16) 牧野貴至, 河野雅樹, 金久保光央, 熱測定, 2017, 44, 85.
- 17) Kasahara, S., Kamio, E., Yoshizumi, A., Matsuyama, H., Chem. Commun., 2014, 50, 2996.
- 18) Moghadam, F., Kamio, E., Yoshizumi, A., Matsuyama, H. Chem. Commun., 2015, 51, 13658.
- 19) Swatloski, R. P., Spear, S. K., Holbrey, J. D., Rogers, R. D., J. Am. Chem. Soc., 2002, 124, 4974.
- 20) Fukaya, Y., Hayashi, K., Wada, M., Ohno, H., Green Chem., 2008,10, 44.
- 21) Abe, M., Fukaya, Y., Ohno, H., Chem. Commun., 2012, 48, 1808
- 22) Kuroda, K., Komori, T., Ishibashi, K., Uto, T., Kobayashi, I., Kadokawa, R., Kato, Y., Ninomiya, K., Takahashi, K., Hirata, E., Commun. Chem., 2020, 3, 163.
Contact Us
E-mail Inquiry Form
Telephone